Abstract
Aim: We investigated the immune effects of nilotinib (NIL) combined with pegylated-interferon-α2b (IFN) in a subgroup of newly diagnosed chronic phase Chronic Myeloid Leukemia (CML) patients within the ALLG CML11 PINNACLE trial. NIL was given for the first 3 months (mo), followed by combination with IFN for 21 mo (from mo 4 - 24), then NIL alone from 25 mo.
Methods: We assessed 12 patients serially from diagnosis, 2, 3, 5, 7, 10, 16 and 28 mo, including samples at 1, 8 and 15 days post-IFN commencement (median of 8 samples per patient). Multi-parameter flow cytometry was used to characterise effector phenotype and function of innate immune Natural Killer (NK) cells, Regulatory T cells (Treg), Myeloid Derived Suppressor Cells (MDSC), CD4+/CD8+ T cells and B cells. Functional cytotoxic T-lymphocyte (CTL) responses to leukaemia-associated antigens (LAAs) WT1, BMI-1, PR3 and PRAME were assessed by interferon-γ (IFN-γ)/tumour-necrosis factor-α (TNF-α) FLUROSPOT using peptide libraries of 15-mer peptides overlapping by 11 amino acids spanning the entire protein, or HLA-A*0201 specific peptides in HLA-A*0201+ patients.
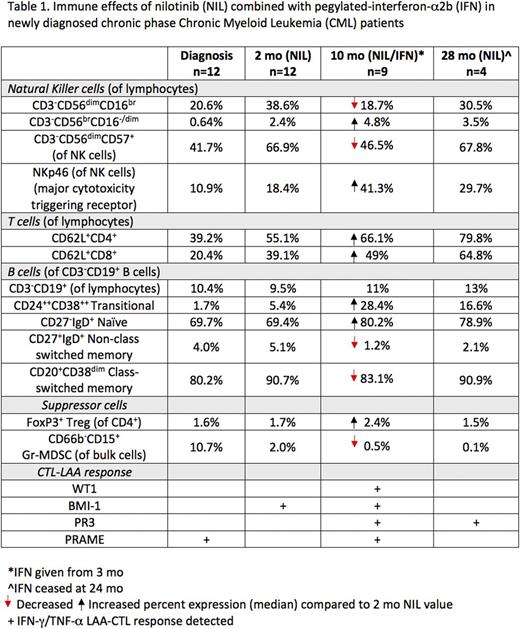
Results: All patients in this cohort had BCR-ABL1 <10% at 3 mo following NIL alone, 91% had optimal BCR-ABL1 responses (<1% and <0.1% at 6 and 12 mo respectively) with NIL/IFN. Cytolytic CD3-CD56dimCD16br NK cells increased after NIL (median; diagnosis 20.6% vs 2 mo 38.6%, p=0.002), the phenotype was more mature (CD57+) with increased CD161, NKp46, and NKp30 receptors. In contrast, the absolute number of CD56dimCD16br NK cells reduced as early as 8 days post-IFN, was lowest at 7 mo (151 cells/μl) on combination treatment vs 2 mo NIL alone (543 cells/μl, p=0.01). NK maturation status also decreased post-IFN and was subsequently restored upon IFN cessation (Table 1). A trend toward increasing immunoregulatory CD56brCD16-/dim NK cells was observed post-IFN (10 mo; 58 cells/μl) vs 2 mo NIL alone (32 cells/μl). NKp30/46 expression increased further on combination therapy, other NK receptors including CD161, KIR2DL2/DL3/DS2 and KIR2DL5 expression and functional degranulation response were unaffected by IFN. Programmed death-1 receptor expression, indicative of immune activation, increased on NK cells post-IFN, but was unchanged in T cells, B cells and monocytes.
Low CD62L which is associated with impaired immune function was observed on CD4+/CD8+ T cells at diagnosis. CD62L surface expression increased from 2 mo NIL and remained increased with NIL/IFN combination. CD62L was highest at 28 mo. Naïve/memory T cell analysis showed decreasing CD8+ but not CD4+ effector memory (EM) cells on combination therapy (10 mo; 13.7%) vs diagnosis (23.3%, p=0.04) and 2 mo NIL (23.9%) with no change in central memory (CM). Functional IFN-γ and/or TNF-α LAA-CTL responses were observed in 83% of patients, with peak responses 15 days post-IFN. PRAME-CTLs (predominantly IFN-γ driven) were the most abundant and detected in all responders, with BMI-1- and PR3-CTLs in 50% of responders.
CD3-CD19+ B cell numbers remained unchanged however, Transitional B cells (CD24++CD38++) the first cells which migrate to the peripheral blood, rapidly increased as early as 15 days post-IFN (283 cells/μl) vs diagnosis (70 cells/μl, p=0.001) and 2 mo NIL (61 cells/μl, p=0.008). CD27-IgD+ Naïve B cells demonstrated a similar increased pattern of expression. In contrast, CD27+IgD+ non class switched and CD20+CD38dim class switched memory B cells decreased following combination therapy.
Suppressor Treg numbers decreased post-NIL vs diagnosis, and increased with IFN. Treg displayed a shift from EM phenotype to CM on NIL, and remained unchanged on combination therapy. T cell immunoreceptor TIGIT+ Tregs representing a highly activated/suppressive phenotype were unchanged at all time points. Predominantly granulocytic (Gr)-MDSC (CD66b+CD15+) were observed at diagnosis, reducing early in patients on NIL alone and remaining low throughout the study period.
Conclusion: This first immunological report of NIL/IFN combination in newly diagnosed CML patients identifies rapid and divergent immunomodulatory effects encompassing both the innate and adaptive immune system. IFN alters NK cell phenotype, without affecting effector function, increases specific early B cell subsets, is associated with enhanced LAA-CTL responses and reduces suppressive Gr-MDSC. Correlation of patient immune responses and clinical outcomes is ongoing.
White: Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Ariad: Consultancy, Honoraria, Research Funding. Yeung: BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Ariad: Honoraria, Research Funding. Hughes: BMS: Consultancy, Honoraria, Research Funding; Ariad: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Yong: Bristol-Myers Squibb: Honoraria, Research Funding; Celgene: Research Funding; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal